Atomic Structure and Interatomic Bonding In Engineering Materials
By the late 19th century, it became evident that many phenomena involving electrons in solids could not be explained using classical mechanics. This led to the development of a set of principles and laws governing atomic and subatomic systems, which later became known as quantum mechanics. Understanding the behavior of electrons in atoms and crystalline solids inevitably involves a discussion of quantum mechanics concepts. However, a detailed exploration of these principles is beyond the scope of this text, and only a simplified and brief overview is provided.
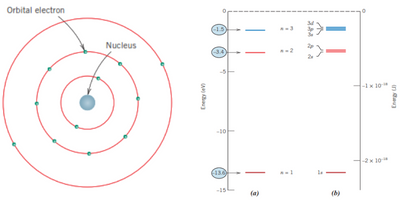
One of the early outcomes of quantum mechanics was the simplified Bohr atomic model, in which electrons are assumed to orbit the atomic nucleus in discrete orbitals, with the specific position of an electron being relatively well-defined in terms of its orbit. The Bohr model was an initial attempt to describe electrons in an atom, addressing both their position (electron orbitals) and energy (quantized energy levels).
However, the Bohr model was found to have significant limitations, particularly its inability to explain certain phenomena involving electrons. These limitations were addressed by the wave-mechanical model, in which electrons are described as exhibiting both wave-like and particle-like characteristics. In this model, electrons are no longer treated as particles moving in fixed discrete orbitals; instead, their position is considered as a probability distribution, representing the likelihood of finding an electron at various locations around the nucleus.
Electron Configuration
To determine the number of atomic subshells filled with orbiting electrons, Pauli’s exclusion principle can be applied, which states that each electron state can hold no more than two electrons, and they must have opposite spins. Therefore, the s, p, d, and f subshells can accommodate, respectively, a maximum of 2, 6, 10, and 14 electrons.
The electron configuration or atomic structure represents the way these subshells are occupied by electrons. In conventional notation, the number of electrons in each subshell is indicated by a superscript after the subshell. For example, the electron configurations for hydrogen, helium, and sodium, based on the periodic table, are 1s¹ (1), 1s² (2), and 1s² 2s² 2p⁶ 3s¹ (11), respectively.
This electron configuration is one of the outputs used to find valence electrons. Valence electrons are electrons that occupy the outermost shell. These electrons are very important as will be seen, they participate in the bonds between atoms to form a group of atoms and molecules. Furthermore, many of the physical and chemical properties are based on these valence electrons that can be observed in the periodic table. The grouping of element groups is also influenced by valence electrons.
INTERATOMIC BONDING
Ionic Bond
Ionic bonding is perhaps the easiest to describe and visualize. This type of bond is found in compounds consisting of metallic and non-metallic elements in the periodic table. Metal atoms easily donate their valence electrons to non-metal atoms. In this process, all atoms achieve a stable or inert gas configuration (i.e., a fully filled orbital shell) and become electrically charged—thus forming ions. Sodium chloride (NaCl) is a classic example of an ionic compound. A sodium atom can assume the electronic structure of neon (with a net positive charge and a smaller size) by transferring its 3s valence electron to a chlorine atom.
Covalent Bond
Covalent bonding occurs in materials where atoms have similar electronegativities and are positioned near each other in the periodic table. In these materials, a stable electron configuration is achieved by sharing electrons between adjacent atoms. Two covalently bonded atoms each contribute at least one electron to the bond, and the shared electrons can be considered to belong to both atoms. Covalent bonds typically occur in compounds made up of non-metal elements.
Van Der Waals Bond
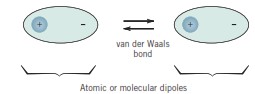
Secondary bonds, or van der Waals (physical) bonds, are weaker compared to primary or chemical bonds; their bond energy ranges from approximately 4 to 30 kJ/mol. Secondary bonds exist between nearly all atoms or molecules but may be overshadowed if one of the three primary bond types (ionic, covalent, or metallic) is present. Van der Waals bonds are evident in inert gases, which have stable electronic structures. In addition, secondary (or intermolecular) bonds can form between atoms or groups of atoms that are already connected by primary (or intramolecular) ionic or covalent bonds.
The van der Waals bonding force arises from atomic or molecular dipoles. Essentially, an electric dipole exists whenever there is a separation of positive and negative charges within an atom or molecule. The bonding results from Coulombic attraction between the positive end of one dipole and the negative region of another nearby dipole. Dipole interactions can occur between induced dipoles, between induced dipoles and polar molecules (which have permanent dipoles), and between polar molecules. Hydrogen bonding, a special type of van der Waals bond, occurs between certain molecules containing hydrogen as one of their elements.
CONTRIBUTOR: Daris Arsyada
Source:
Callister, William D. Jr, dan Rethwisch, David G. 2018. Materials Science and Engineering An Introduction (10th ed). Amerika Serikat: John Wiley & Sons, Inc.